On August 3, 2020, the Centers for Medicare & Medicaid Services (CMS) released the CY 2021 Revisions to Payment Policies under the Physician Fee Schedule and Other Changes to Part B Payment Policies [CMS-1734-P], which includes proposals related to Medicare physician payment and the Quality Payment Program (QPP). Since the public health emergency (PHE) was declared earlier this year, the Administration has issued waivers to increase flexibility and reduce regulatory burden to help providers meet the demands of the Coronavirus (COVID-19) pandemic. This rule includes several proposals to make permanent, extend or transition out of these COVID-19 flexibilities.
Comments on the proposed rule are due on October 5, 2020. CMS indicated that it will waive the 60-day publication requirement for the Final Rule and replace it with a 30-day notification. This means that the Final Rule will be effective January 1, 2021, even though it may not be published until December 1, 2020, instead of the typical November 1 target publication date.
Read on for a topline summary of the major provisions in the proposed rule.
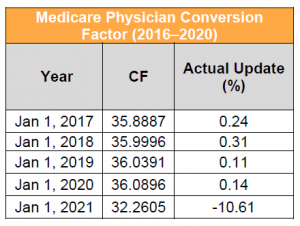
Medicare physician payment is based on the application of a dollar-based conversion factor (CF) to work, practice expense (PE) and malpractice relative value units (RVUs), which are then geographically adjusted. Work RVUs capture the time, intensity and risk of the provider; PE RVUs capture the cost of supplies, equipment and clinical personnel wages used to furnish a specific service; and malpractice RVUs capture the cost of malpractice insurance.
Key Takeaway: CY 2021 CF Would Decrease to $32.2605, a Reduction of More Than 11%
The 2021 proposed physician CF is $32.2605. This represents a decrease of $3.83 from the 2020 conversion factor of $36.0896. The proposed 2021 anesthesia CF is $19.9631, in comparison to the 2020 CF of $22.2016. This proposed negative adjustment results from a statutorily mandated budget neutrality adjustment to account for changes in work RVUs. The change in work RVUs is driven largely by updates to evaluation and management (E/M) services that were finalized in the CY 2020 Physician Fee Schedule (PFS) Final Rule, but that are not effective until January 1, 2021, as well as other proposed changes in work. Stakeholders are urging Congress to suspend the budget neutrality adjustment for E/M changes effective CY 2021 and are encouraging CMS to use its authority to avoid the reduction. Some stakeholders are urging Congress to suspend the budget neutrality adjustment for E/M changes effective CY 2021.
In 2021, certain clinicians would also be subject to an additional +/- 7% payment adjustment based on their performance in the 2019 performance year of Merit-based Incentive Payment System (MIPS) of the QPP.
Key Takeaway: Impact by Specialty Ranges from -11% to +17%
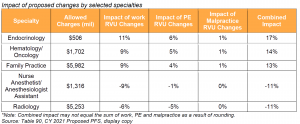
Actual payment rates are affected by a range of proposed policy changes related to physician work, PE and malpractice RVUs. CMS summarizes these changes in Table 90 in the proposed rule. Impact by practice would vary based on service mix. Specialty impacts range from -11% for radiology and nurse anesthetists/anesthesiologist assistants, to +17% for endocrinology. The proposed range of impact by specialty has increased substantially relative to previous rulemaking cycles. In the CY 2020 PFS, for example, impact by specialty ranged from -4% to +3%.
While some of the differences in specialty impact result from proposed changes to individual codes, the wide range in specialty impact is largely due to E/M payment changes slated to begin in 2021 and the statutory requirements around budget neutrality. Effective January 1, 2021, CMS will implement payment rate increases for office/outpatient E/M codes and simplified coding and billing requirements for E/M visits, which CMS estimates will save clinicians 2.3 million hours per year in burden reduction. CMS finalized this policy in the CY 2020 PFS Final Rule but delayed implementation until 2021. Specialties that do not generally bill office/outpatient E/M visits would experience the greatest decreases, while specialties and practices that bill higher level established patient visits would see the greatest increases, as those codes were revalued higher relative to the rest of the office/outpatient E/M code set.
If Congress acts to waive budget neutrality or otherwise dull the effect thereof, the specialty level impacts could change dramatically.
Key Takeaway: CMS Continues to Implement Updated PE Input Pricing
For CY 2019, CMS worked with market-research company StrategyGen to conduct an in-depth and robust market research study to update the PFS direct PE inputs (DPEI) for supply and equipment pricing. Through this initiative, CMS updated the pricing for more than 2,000 supply and equipment items used as DPEI. CMS is phasing in the new pricing over four years. CY 2021 is the third year of the transition, which means that PE input pricing for the affected items in 2021 will be based on 75% of the new pricing and 25% of the old pricing. Given the large number of affected codes, it is important for stakeholders to monitor how the updated pricing might affect proposed payment. CMS will considers invoices submitted as public comments during the comment period following the publication of the PFS proposed rule, so stakeholders should submit invoices during the public comment period to request updates to pricing.
Key Takeaway: CMS Solicits Comments on Potential Refinements to PE Methodology
The RAND Corporation is currently studying potential improvements to CMS’s PE allocation methodology and the data that underlie it. RAND convened a technical expert panel to obtain input from stakeholders, including physicians, practice and health system managers, healthcare accountants and health policy experts. Based on the results of the panel’s work and RAND’s other ongoing research, CMS might refine the PE methodology and update the data used to make payments under the PFS.
CMS is considering several questions, including how to best incorporate market-based information, which could be similar to the market research that CMS recently conducted to update supply and equipment pricing used to determine direct PE inputs under the PFS payment methodology. For example, CMS solicits comments on how it might update the clinical labor data. Historically CMS has used data from the US Bureau of Labor Statistics, and is seeking comments to determine if this is the best data source or if there is a better alternative. CMS is also interested in hosting a town hall meeting, at a date to be determined, to provide an open discussion with stakeholders on its ongoing research to potentially update the PE methodology and the underlying inputs.
CMS invites feedback from all interested parties regarding RAND’s report, and is not making any proposals based on this report at this time. Stakeholders are encouraged to submit feedback as part of their public comments or, if outside the public comment process, via email at PE_Price_Input_Update@cms.hhs.gov.
Key Takeaway: CMS Proposes to Expand Telehealth Access Post-PHE
One of the key changes implemented through COVID-19 waivers was expanded flexibility for telehealth services, which has led to an uptick in the use of telehealth, remote patient monitoring and communication-technology-based services. While the PHE expanded the Secretary’s authority to provide telehealth flexibilities, in normal circumstances CMS’s authority to make changes is mainly limited to the list of services approved for telehealth coverage. In this proposed rule, CMS seeks public input on expanding the number of services available to Medicare beneficiaries through telehealth capabilities.
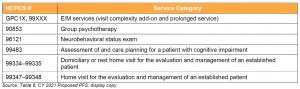
Key Takeaway: CMS Proposes to Add Services Similar to Codes Currently on the Medicare Telehealth List
CMS seeks comment on adding the codes listed below to the Medicare telehealth list.
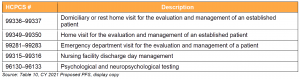
Key Takeaway: CMS Proposes to Temporarily Add Services to the Medicare Telehealth List
CMS seeks public input on temporarily including a select number of services that were added during the PHE for which there is likely to be clinical benefit when furnished via telehealth, but where there is currently insufficient evidence to permanently add these services. CMS proposes that these services would remain on the list of approved telehealth services through the calendar year in which the declared COVID-19 PHE ends.
Key Takeaway: CMS Seeks Input on Coding and Payment Policy for Audio-Only Services
CMS established separate payment for audio-only telephone E/M services during the PHE and cross-walked payment rates for these services from office/outpatient E/M codes. CMS has stated that it does not have the authority to extend this flexibility beyond the PHE because statute requires telehealth services to have a two-way, audio/video communication technology. In the proposed rule, CMS acknowledges the value of audio-only services and seeks comments on whether CMS should develop coding and payment for a service similar to the existing virtual check-in codes but for a longer unit of time and with an accordingly higher value.
CMS also seeks comments on several other telehealth-related proposals.
CMS also proposes to make a technical revision to the telehealth list by removing six Health and Behavior Assessment and Intervention procedure CPT codes that were deleted from CPT and replacing them with the nine new successor codes.
On the same day that the proposed rule was released, President Trump signed an Executive Order on Improving Rural Health and Telehealth Access, which included proposals to expand telehealth access in rural communities and improve rural improve telecommunications infrastructure. This is an indication of the Administration’s continued focus on this issue.
Key Takeaway: CMS Proposes Updates and Clarifications for Remote Physiologic Monitoring Services
In recent years, CMS has established payment for several remote physiologic monitoring (RPM) codes. During the PHE, CMS implemented certain flexibilities around these services. In response to stakeholder questions, the proposed rule clarifies existing policy for RPM codes and proposes a new permanent policy.
CMS clarifies that following the PHE, it will again require that an established patient-physician relationship exist for RPM services to be furnished. CMS also clarifies policies around medical devices, stating that only physicians and nonphysician practitioners who are eligible to furnish E/M services may bill RPM services, and that practitioners may furnish RPM services to patients with acute conditions as well as patients with chronic conditions. CMS clarifies the definition of “interactive communication,” and that RPMs are E/M services.
CMS proposes as permanent policy to allow providers to obtain consent at the time that RPM services are furnished, and to allow auxiliary personnel to furnish CPT code 99453 and 99454 services under a physician’s supervision. Auxiliary personnel include contracted employees.
Finally, CMS solicits comments on whether the current RPM codes accurately and adequately describe the full range of clinical scenarios where RPM services may be of benefit to patients.
Key Takeaway: CMS Solicits Comments on E/M Add-On Code for Complexity, GPC1X
In the CY 2020 PFS Final Rule, CMS established add-code GPC1X for office/outpatient E/M visit complexity with an effective date of CY 2021. Since the code was established, CMS has received stakeholder feedback that the code definition is unclear, as are the rules on when it is appropriate to report the code. Stakeholders have also expressed concerns regarding CMS’s utilization assumptions that specialties that predominantly furnish the kind of care described by the code would bill it with every visit. Utilization assumptions are meaningful since they affect the overall budget neutrality adjustment and, in turn, the physician CF.
In this proposed rule, CMS requests more specific information regarding what aspects of the definition of HCPCS add-on code GPC1X are unclear, how they might address those concerns, and how they might refine their utilization assumptions for the code.
Key Takeaway: CMS Proposes Increased Payment for Bundled Services
As noted, CMS will increase payment rates for office and outpatient E/M visits beginning in 2021. In this rule, CMS proposes similar increases to the value of many bundled services that are comparable to or include office/outpatient E/M visits. These proposed payment increases would implement recommendations from the American Medical Association RVS Update Committee. These bundled services include:
Key Takeaway: CMS Proposes Reducing Supervision Requirements
The Medicare program has traditionally had a mix of requirements for the level of training necessary for services to be covered and paid. Some services defer to state scope of practice laws, while others have specific, more restrictive Medicare requirements. In 2019, Executive Order 13890 sought to lighten restrictions imposed by Medicare. CMS has since eased some restrictions as part of the flexibilities it created for the PHE. The proposed rule seeks to make some of these easements permanent.
CMS has previously restricted services provided by physicians-in-training and resident physicians from being directly billed to Medicare. Services furnished by these physicians-in-training could be billed as a service of a supervising teaching physician, given that the teaching physician was physically present for key parts of the encounter. During the PHE, CMS permitted several flexibilities to these supervision requirements, including allowing teaching physicians to provide virtual supervision using telecommunications technology, permitting inpatient services rendered by resident physicians at their training institutions to be covered by Medicare, and allowing higher complexity E/M services in primary care centers to be rendered without the teaching physician’s presence.
Prior to the PHE, generally only physicians could supervise the performance of diagnostic tests. While non-physician practitioners could perform many diagnostic tests if permitted by the state in which they practice, only physicians could supervise those personnel performing the tests. CMS relaxed this requirement during the PHE and is now considering making the change permanent.
The proposed rule clarifies that pharmacists may be considered auxiliary personnel and assist with medication management when their services are billed to Medicare Part B and rendered incident to a physician’s service. This is not a regulatory change on the part of CMS, but rather a clarification of the current regulatory requirements, according to the agency.
The proposed rule also seeks comment on permitting therapy assistants (physical and occupational) to provide maintenance therapy on an outpatient basis. Medicare has previously clarified that maintenance therapy is covered if a patient has a skilled need for therapy. For patients receiving maintenance therapy in a skilled nursing facility or in a home health episode of care, therapy assistants are permitted to provide maintenance therapy. Under the PHE, CMS permitted assistants to do so in the outpatient setting as well. CMS is not considering making such a change permanent at this time.
CMS seeks public comment on the risks and benefits of making the above changes permanent. CMS is weighing considerations such as the effect on utilization, quality of care and access to care. The net effect remains unclear to the agency, as there is generally a reduced reimbursement for services provided to individuals with a lower level of training. Therefore, whether potential increased utilization will be offset by lower reimbursement is uncertain.
Finally, CMS also seeks comments on proposed policies related to medical record documentation, PFS payment for teaching physicians, and direct supervision by interactive telecommunications technology.
Key Takeaway: CMS Solicits Comments on Potentially Misvalued Code
The Affordable Care Act mandates regular review of fee schedule rates for physician services paid by Medicare, including services that have experienced high growth rates. CMS established the Potentially Misvalued Code process to meet this mandate. In the CY 2021 proposed PFS, CMS proposes one code for review under this process and requests public comment on this code. This code was identified by a public nomination process directly through CMS:
Key Takeaway: CMS Proposes Updates to TCM and Opioid Treatment Services
TCM Codes (99495, 99496)
Effective January 1, 2013, CMS established TCM codes (99495, 99496) to report physician or qualifying nonphysician practitioner care management services for a patient following a discharge from a hospital; skilled nursing facility; or community mental health center, outpatient observation or partial hospitalization. At the time these codes were established, CMS identified 57 codes that cannot be billed simultaneously because of potential duplication of services. In CY 2020 Final Rule, CMS noted that utilization of the TCM services was low and believed that increased utilization of medically necessary TCM services could improve patient outcomes. In CY 2020, CMS removed 16 services from the list of codes that could not be billed concurrently with TCM services.
In the proposed rule, CMS identifies 15 additional codes that can be billed concurrently with TCM, including 14 codes for end-stage renal disease services and one complex chronic care management service. Table 14 in the proposed rule provides a comprehensive lists of these codes.
As noted, CMS also proposes to increase the value of TCM codes in 2021.
Opioid Use Disorder (OUD) Treatment
Building on proposals for opioid use disorder (OUD) treatment in the CY 2020 PFS, CMS proposes to add naloxone to the definition of OUD treatment services to increase access to this critical therapeutic. To account for the additional cost for these drugs, CMS proposes to adjust payment for OUD services through two new add-on codes: GOTP1 and GOTP2. CMS proposes payment rates for these new add-on codes will be established through the same process used to price the bundled OUD services. Table 30 in the proposed rule provides a summary of the proposed add-on codes.
Key Takeaway: CMS Proposes to Remove Nine NCDs
CMS seeks comments on its proposal to remove nine NCDs. CMS believes that these NCDs may no longer contain pertinent or clinically relevant information and are rarely used by beneficiaries. Services that were automatically covered under these NCDs would now be covered at the Medicare Administrative Contractor’s (MAC’s) discretion, and coverage of services that were previously barred by the NCDs is also at the discretion of the MAC. CMS believes this will result in greater contractor flexibility and will better serve the needs of Medicare beneficiaries.
Proposed Transition of NCDs from Limited Coverage to MAC Discretion
Proposed Removal of Non-Covered NCDs
Under the QPP, eligible clinicians will elect either to be subject to payment adjustments based upon performance under MIPS or to participate in the Advanced Alternative Payment Model (APM) track. Eligible clinicians choosing the MIPS pathway will have payments increased, maintained or decreased based on relative performance in four categories: Quality, Cost, Promoting Interoperability (meaningful use) and Improvement Activities. Eligible clinicians choosing the APM pathway will automatically receive a bonus payment once they meet the qualifications for that track.
Because of the COVID-19 pandemic, CMS has limited QPP proposals to only the highest priorities, in order to continue to advance policy goals of the program while being sensitive to providers on the frontlines of pandemic response.
Key Takeaway: CMS Would Delay MVP Implementation
Currently, implementation of the MIPS Value Pathway (MVP) framework is slated to begin on January 1, 2021. CMS proposes to delay implementation until the 2022 performance period, or later. The MVP was designed to motivate clinicians to move away from reporting on disconnected activities and measures, and towards an aligned set of measure options that are meaningful to patient care and more relevant to a clinician’s scope of practice.
In the proposed rule, CMS updates the five guiding principles when developing MVPs to reflect comments from stakeholders, and includes an additional guiding principle to support the transition to digital quality measures. The proposed rule also outlines an MVP development process in which CMS emphasizes the importance of including patient perspectives, and describes the process through which stakeholders can submit MVP candidates.
Key Takeaway: CMS Introduces the APM Performance Pathway
To reduce provider reporting burden for MIPS-eligible clinicians who are already participating in an APM, CMS proposes the APM Performance Pathway (APP). This pathway would provide data reporting consistency for participants in MIPS APMs. The proposed implementation date for the APP is January 1, 2021.
CMS proposes sunsetting the CMS Web Interface as a collection type beginning in the 2021 performance period.
Key Takeaway: CMS Lowers Previously Finalized Threshold for Avoiding Negative Payment Adjustments for the 2021 MIPS Performance Year
The proposed MIPS performance threshold for the 2021 performance year is 50 points (10 point reduction from the target finalized for CY 2021 in the CY 2020 Final Rule). To avoid a negative adjustment, providers must reach this performance threshold.
CMS proposes to reduce the weight of the Quality Performance Category from 45% to 40% and increase the weight of the Cost Performance Category from 15% to 20%.
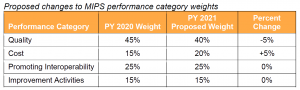
Key Takeaway: CMS Proposes to Increase Performance Year 2020 Complex Patient Bonus Points to Account for COVID-19
CMS proposes to change the maximum number of points available for the complex patient bonus to 10 points to account for the additional complexity of treating COVID-19 and other patients during the PHE. CMS proposes the increase for the 2020 performance period only.
Key Takeaway: CMS Would Modify Medicare Accountable Care Organization (ACO) Quality Reporting and Performance Measurement
CMS proposes to implement the APP for MIPS APMs for MSSP ACOs for the 2021 performance year. The agency intends for this change to reduce burden, align measures across programs and address high priority areas for quality improvement. Under the new approach, ACOs would report one set of quality measures that would satisfy MSSP and MIPS requirements. The measures set would be smaller, a decrease from 21 measures to six. CMS also proposes to increase the quality performance standard for ACOs, except those eligible for facility-based scoring. Meeting the quality performance standard would be tied to an ACO’s shared savings and shared loss potential.
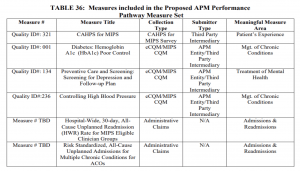
Beyond the quality updates, CMS proposes modifications to the definition of primary care services that are used for beneficiary alignment to an ACO. These changes take into account some of the feedback from stakeholders around the use telehealth services. CMS also proposes modifications to the extreme and uncontrollable circumstances policies. ACOs should carefully review these proposals and are encouraged to provide feedback on the proposed additions, deletions and other services that should be included in the future.
Key Takeaway: CMS Proposes Adjustments to Advanced APM Bonus Payments Processes, but Omits Changes Requested by the Stakeholder Community
CMS proposes a change to the calculation of Threshold Scores used to make Qualifying APM Participant (QP) determinations. For the 2021 participation year, Medicare patients who are prospectively attributed to an APM entity would not be included in the denominator for the APM threshold calculation. The proposed rule includes provisions that clarify calculation of the bonus payment and create a step-wise hierarchy for bonus payments in cases where tax identification numbers with which the QP has an association cannot be identified. CMS also proposes to establish a targeted review process for QP or partial QP determinations if the APM entity or eligible clinician believes in good faith that an eligible clinician was omitted from a Participation List because of a CMS clerical error. Decisions based on the targeted review would remain final; there would be no further administrative or judicial review.
CMS also clarifies that changes to shared savings and losses as a result of the COVID-19 pandemic would not jeopardize advanced APM determinations for models previously determined to be Advanced APMs. This is consistent with previous CMS guidance to ACOs. CMS clarifies that provisions of the Participation Agreements or governing regulations in response to the PHE would not be considered to the extent that they would prevent an APM from meeting the criteria for a year.
Stakeholders have called on CMS to make changes to the advanced APM bonus payment and eligibility criteria, including modifying the patient count thresholds for advanced APM qualification, setting a date by which bonus payments will be made, and providing greater transparency around bonus calculations. The proposed rule does not provide relief on these points.
Immunization Services
Part B Drug Payment for Drugs Approved Under Section 505(b)(2) of the Food, Drug, and Cosmetic Act
Federally Qualified Health Centers (FQHC) Market Basket
For more information, contact Paul Gerrard, Kelsey Haag, Sheila Madhani, Mara McDermott or Christine Song.